This week's case was generously donated by Dr. Manohar Mutnal. The following were seen in a peripheral blood smear from a patient with an unknown travel history. What is your differential diagnosis? What additional information would you like?
Monday, June 30, 2025
Sunday, June 29, 2025
Answer to Case 780
Answer to Parasite Case of the Week 780: Trypanosoma brucei trypomastigotes.
As noted by Florida Fan, "This is definitely a case of trypanosomiasis. The flagellate doesn’t show a prominent kinetoplast nor assume a C shape in general. This rules out Chagas disease caused by T. cruzi. We have Trypanosoma brucei, yet morphology alone doesn’t warrant a differential diagnosis of subspecies gambiense nor rhodesiense."
Idzi also noted that "In the first picture we can clearly see the difference between the two morphologies of T. brucei: the "short stumpy" form (adapted for survival in the tsetse fly vector --> transmission) versus the "long slender" form (which multiplies in the host)!" Here is an annotated version of this image showing these two morphologies:
I had also asked what additional information is needed in this case - and you all responded with excellent suggestions. In summary,
- We first, we need to know the travel history to determine the likely subspecies. PCR could also be performed. This is important for treatment and prognostic implications.
- Second, we need to know the stage of disease, as this will also drive treatment decisions. As noted by Idzi, "A lumbar puncture will be able to tell us if the patient has evolved to stage II of the disease, where the parasite has invaded the central nervous system. Even if no tryps are found in the CSF, a raised number of WBCs in the CSF will still be indicative of stage II disease (when tryps are found in the blood).
Monday, June 16, 2025
Case of the Week 779
This week's case was generously donated by Dr. Richard Bradbury. A patient living in The Gambia presented with high fever, body aches, and altered consciousness. Images from the Giemsa-stained thick and thin blood films are shown below.
Due to a shortage of coartem, quinine was administered. Shortly afterwards, the patient's urine turned dark brown:
What is this condition, and what is it caused by?
Sunday, June 15, 2025
Answer to Case 779
Answer to the Parasite Case of the Week 779: "Black Water fever" - a massive hemolytic event associated with P. falciparum infection and quinine administration.
Black water fever was previously an important cause of death and was prominently reported in British soldiers in the early 20th century. Thankfully, it is rarely seen today with the advent of synthetic antimalarials (e.g., chloroquine) and artemisinin combination therapies. The exact etiology is poorly understood, and seems to be attributed to a complex interaction between the host RBC, the parasite, and antimalarial drugs. It may also occur more often in people with G6PD deficiency.
Tuesday, June 10, 2025
Case of the Week 778
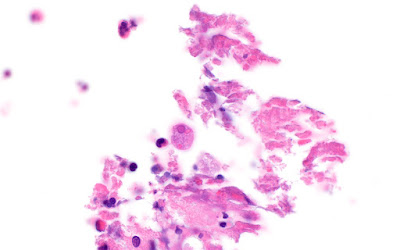
This week's case features the intestinal biopsy of a middle aged man with abdominal pain and diarrhea. The astute pathologist noted these small objects (~20 microns in greatest dimension) associated with ulcerated colonic mucosa. Stain is hematoxylin and eosin (10x, 40x, and 100x objectives). What is your diagnosis?
Sunday, June 8, 2025
Answer to Case 778
Answer to the Parasite Case of the Week 778: Amebiasis due to Entamoeba histolytica.
As noted by Dr. Jacob Rattin, "It looks like there is ulceration in the adjacent mucosa and Entamoeba histolytica trophozoites with visibly ingested red blood cells." Several others also noted the ingested RBCs within the trophozoite cytoplasm.
When seen in stool specimens, the presence of RBCs within Entamoeba trophozoites allows us to presumptively call this E. histolytica rather than one of the identical-appearing amebae such as E. dispar. However, in this case, we have another important clue that allows us to presumptively identify the ameba, even if we don't see ingested RBCs: the presence of trophozoites associated with or invading into the ulcerated mucosa. E. dispar is not considered a pathogen, and other doppelgangers (e.g., E. moshkovskii, E. bangladeshi) have not been definitively shown to be pathogenic. Thus, the presence of invasive Entamoeba trophozoites points us towards E. histolytica. Note that the trophozoites look somewhat different in tissue than they do in stool as the central chromatin dot is often not present. However, the outer rim of chromatin is easily visible.
Sunday, May 18, 2025
Case of the Week 777
Dear Readers,
I am excited to announce that we are celebrating our 777th case!
In honor of this milestone, we have a selection of 3 helminth eggs for you to identify. You win the parasite jackpot if you can get all three. There is an 'easy' and 'hard' version, so you get to take your pick.Answer to Case 777
The Answer to the Parasite Case of the Week 777 is up - and it's a jackpot of parasite eggs!
Easy version:
- Trichuris trichura
- Ascaris lumbridoides
- Taenia sp.
Hard version:
- Bertiella sp.
- Acanthocephalan egg (M. moniliformis - expressed from the worm so slightly immature, which is why it's not as clear as you would expect)
- Inermicapsifer or Raillietina (Actually Inermicapsifer, but as Idzi mentioned, you cannot tell the egg capsules apart from these two).
How many of you got the jackpot?!?
Thank you for playing for more than 18 years and 777 cases 😉
Saturday, May 17, 2025
Answer to Case 776
Answer to the Parasite Case of the Week 776: Toxoplasma gondii tachyzoites. As the name implies, the tachyzoite is the rapidly dividing stage of the parasite (tachy is from the Greek takhus meaning rapid, swift). Tachyzoites invade cells and divide rapidly within parasitophorous vacuoles, ultimately rupturing infected cells. Tachyzoites divide by endodyogeny, an interesting form of replication seen with some coccidia in which two daughter cells develop internally within the parent cell without nuclear conjugation. The parent cell is consumed in the process - yikes!
Shown here is the classic arc-shaped tachyzoite and 2 daughter cells resulting from the process of endodyogeny:
Monday, May 12, 2025
Case of the Week 776
This week's case is a brain biopsy from a middle-aged man with untreated HIV. The specimen appeared necrotic and bloody. Touch preps were made from the material and stained with Giemsa. From the images below taken with the 100x oil objective, what is your diagnosis?