Dr. Rosenbaum was made aware of this case when he was paged by a neurosurgeon. As a clinical pathologist, he was quite surprised to be asked to come to the operating room; however, he soon understood the request when he saw what was incidentally noted on the patient's skin during brain surgery:
Monday, December 31, 2018
Case of the Week 525
This week's case is an 'oldy but goody' - a case donated to me by Dr. Eric Rosenbaum years ago that I just re-discovered. The history is particularly fun, and a great way to end 2018.
Dr. Rosenbaum was made aware of this case when he was paged by a neurosurgeon. As a clinical pathologist, he was quite surprised to be asked to come to the operating room; however, he soon understood the request when he saw what was incidentally noted on the patient's skin during brain surgery:
This little arthropod was quite active and rather hard to catch (!) The patient is from the southeastern United States. Identification?
Dr. Rosenbaum was made aware of this case when he was paged by a neurosurgeon. As a clinical pathologist, he was quite surprised to be asked to come to the operating room; however, he soon understood the request when he saw what was incidentally noted on the patient's skin during brain surgery:
Sunday, December 30, 2018
Answer to Case 525
Answer: Amblyomma sp. nymph
Everyone did a great job describing the diagnostic features of this tick. There were a few that were trying to determine the gender of the tick, but as Blaine reminded us, it is not possible to determine the gender of a nymph - both male and females will have a short dorsal shield. Below are some of the key morphologic features that are useful in this case.
This constellation of features is consistent with an Amblyomma species. You would need to have a better view of the hypostome (absent in this case) and coxal spurs in order to make it to a species-level identification.
Old One made an interesting comment which I hadn't heard before: "The broken hypostome and chelicera hints at the possibility that this tick has long mouthparts that were cemented deep in the host's tissue. Ticks with shorter mouth parts (e.g. Dermacentor) cement at the surface of the skin, often leaving a skirt of cement attached to the capituli (may be confused as host skin) and often retain their mouthparts." He also noted "The high activity level of this tick is a characteristic seen in Amblyomma and even more so in its cousin Hyalomma. They are sometimes described as almost predatory. Literally one can hear them running through grass to get to their host (prey)." This is behavior I have witnessed first hand, and am dreading the day that Amblyomma americanum makes its way up into Minnesota!
If you are looking for resources on tick identification, you can start with some easy guides such as the ones that Blaine and I published (CAP arthropod bench reference guide, and our CMR article "Laboratory Identification of Arthropod Ectoparasites"). These contain references for species-specific keys as well. There is also a nice key in Lynn Garcia's text and the Manual of Clinical Microbiology chapter on arthropods. Georgia Southern University also has a fun interactive key that walks you though some of the main decision points for tick identification. Happy tick hunting!
Everyone did a great job describing the diagnostic features of this tick. There were a few that were trying to determine the gender of the tick, but as Blaine reminded us, it is not possible to determine the gender of a nymph - both male and females will have a short dorsal shield. Below are some of the key morphologic features that are useful in this case.
- The tick has 8 legs, thus indicating that it is either a nymph or adult (vs. a larva).
- There is no porose area or genital aperture, indicating that it is a nymph.
- The anal groove is posterior to the anus (some call the groove "chalice-shaped"), thus eliminating Ixodes spp.
- The basis capituli does not have lateral projections (i.e. it is rectangular rather than hexagonal), thus ruling out Rhipicephalus spp.
- The palpi are longer than the basis capituli, thus ruling out Dermacentor and Haemaphysalis species.
- It has eyes, thus ruling out Haemaphysalis and Ixodes species.
- There are festoons present, although they are difficult to see. These are seen with Amblyomma, Dermacentor, and Haemaphysalis species, but not Ixodes spp.
This constellation of features is consistent with an Amblyomma species. You would need to have a better view of the hypostome (absent in this case) and coxal spurs in order to make it to a species-level identification.
Old One made an interesting comment which I hadn't heard before: "The broken hypostome and chelicera hints at the possibility that this tick has long mouthparts that were cemented deep in the host's tissue. Ticks with shorter mouth parts (e.g. Dermacentor) cement at the surface of the skin, often leaving a skirt of cement attached to the capituli (may be confused as host skin) and often retain their mouthparts." He also noted "The high activity level of this tick is a characteristic seen in Amblyomma and even more so in its cousin Hyalomma. They are sometimes described as almost predatory. Literally one can hear them running through grass to get to their host (prey)." This is behavior I have witnessed first hand, and am dreading the day that Amblyomma americanum makes its way up into Minnesota!
If you are looking for resources on tick identification, you can start with some easy guides such as the ones that Blaine and I published (CAP arthropod bench reference guide, and our CMR article "Laboratory Identification of Arthropod Ectoparasites"). These contain references for species-specific keys as well. There is also a nice key in Lynn Garcia's text and the Manual of Clinical Microbiology chapter on arthropods. Georgia Southern University also has a fun interactive key that walks you though some of the main decision points for tick identification. Happy tick hunting!
Monday, December 24, 2018
Case of the Week 524
Happy Holidays to all of my readers and fellow parasitologists! Here is a little holiday cheer by way of a photo and poem from Blaine:
Dashing through the fur
With modified jumping legs
O’er the hair we go
Spreading Yersinia pestis all the way! Mwa ha ha
Piercing and sucking mouthparts
Make for a painful bite!
Oh what fun it is to spread
This deadly pandemic blight!
Oh, Jingle Bugs Jingle Bugs
Jingle All the Way
Oh what fun it is to spread
Yersinia pestis all the way!
Oh, Jingle Bugs Jingle Bugs
Jingle All the Way
Oh what fun it is to spread
Yersinia pestis all the way!
Monday, December 17, 2018
Case of the Week 523
I have a bit of a puzzle for you this week. The following are transmission electron microscopy (TEM) images of a liquid-based stock culture that we used to keep refrigerated for use as a positive control. This positive control organism was plated at the same time as patient specimens to ensure that our culture media was capable of sustaining the organism's growth. Cultures were accomplished by plating the control or the patient specimen onto a non-nutrient agar overlain with a lawn of bacteria as a food source. We recently did away with this culture in favor of a faster real-time PCR assay that is performed daily (cultures used to take up to 5 days to become positive). As someone who loves microbe morphology, I decided to preserve this organism forever by submitting our stock culture for TEM analysis. Here are some representative images that we took:
What is this organism?
What is this organism?
Sunday, December 16, 2018
Answer to Case 523
Answer: Acanthamoeba sp. cysts and trophozoite.
Wow, I really enjoyed reading the comments on this case! If you haven't already, I encourage you all to ready the holiday tale of Ascaris suum (a.k.a. Ascaris lumbricoides) by Old One. The story confirmed my suspicions of why we see so many cases of human ascariasis in patients from the Midwestern United States who haven't traveled internationally. The pigs are loaded with them!
The comments also nicely describe the differences between the cysts and trophozoites of the free-living amebae that most commonly cause human disease: Acanthamoeba species, Balamuthia mandrillaris, and Naegleria fowleri. Therefore I won't describe them further here. However I will show you some of the main discernible features from the transmission electron micrographic images in this case:
Wow, I really enjoyed reading the comments on this case! If you haven't already, I encourage you all to ready the holiday tale of Ascaris suum (a.k.a. Ascaris lumbricoides) by Old One. The story confirmed my suspicions of why we see so many cases of human ascariasis in patients from the Midwestern United States who haven't traveled internationally. The pigs are loaded with them!
The comments also nicely describe the differences between the cysts and trophozoites of the free-living amebae that most commonly cause human disease: Acanthamoeba species, Balamuthia mandrillaris, and Naegleria fowleri. Therefore I won't describe them further here. However I will show you some of the main discernible features from the transmission electron micrographic images in this case:
Monday, December 10, 2018
Case of the Week 522
This week's case features a whole slide image (WSI) of an H&E-stained section from a bladder resection. You can access the WSI HERE. Like the other WSIs that I've features on my blog, you don't need any special software or a log-in username to access the image. You can easily use the track ball on your mouse to zoom in and out. If using a smart phone or tablet, you can pinch in or out to zoom. Enjoy! (P.S. there are 2 things of interest on this slide).
Sunday, December 9, 2018
Answer to Case 522
Answer to Case 522: Schistosoma haematobium eggs and adults.
Kudos to those of you who saw both the eggs and the adults! I realize that many parasitologists are not used to seeing formalin-fixed paraffin-embedded tissue sections, so here is an overview of the tissue and the primary findings:
Here are some cross-sections of our schisto couple - the male forming the outer part of the 'embrace' and the female being on the inside. Another one of my microbiology instructors likened this relationship to a hot dog and a bun. The male is the bun and the female is the hot dog.
The black pigment within the female is hemozoin formed through breakdown of the ingested host hemoglobin. This is the only parasite other than Plasmodium to form hemazoin pigment in humans. Nine years ago I posted a CASE of the male and female worms together which may help you better visualize their configuration.
Kudos to those of you who saw both the eggs and the adults! I realize that many parasitologists are not used to seeing formalin-fixed paraffin-embedded tissue sections, so here is an overview of the tissue and the primary findings:
This full-thickness section of bladder wall shows a very inflamed mucosa (epithelium and lamina propria) with numerous Schistosoma eggs. Most pathologists would immediately pick out the eggs - each with a thin outer shell and internal miracidium.
However, what many may not have noticed was that the culprits of this disease were also in the picture! Adult male and female schistosomes were residing within the bladder venules and can be seen in this section. As Old One mentioned, "The embrace (thanks eob) of the female by the male is unique to the "Schistos" for a couple reasons. Schistosomes derives from Greek meaning split of cleft body. This particular cleft is a split between male and female reproductive organs, making it dioecious (separate sexes) where other flukes are monoecious (both sexes in one individual). The small size of female to male is the second unique feature. This allows the female to break the embrace and swim down the smaller capillaries to deposit its eggs closer to the mucosal surface than if it were in a bulkier monoecious form, thereby being closer to the external environment. Improving the survival of the egg." Fascinating biology! I was told by one of my instructors years ago that the female usually came back to the same male after her egg-laying, but if she encountered another available male on her way back, she may stay with him instead (!)Here are some cross-sections of our schisto couple - the male forming the outer part of the 'embrace' and the female being on the inside. Another one of my microbiology instructors likened this relationship to a hot dog and a bun. The male is the bun and the female is the hot dog.
The black pigment within the female is hemozoin formed through breakdown of the ingested host hemoglobin. This is the only parasite other than Plasmodium to form hemazoin pigment in humans. Nine years ago I posted a CASE of the male and female worms together which may help you better visualize their configuration.
Monday, December 3, 2018
Case of the Week 521
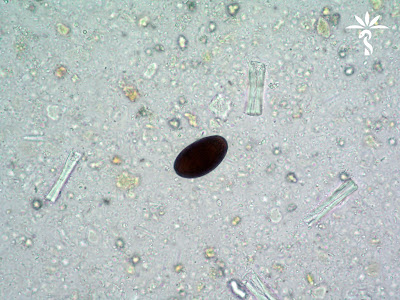
This month's case from Idzi Potters and the Institute of Tropical Medicine, Antwerp, features a stool specimen from a traveler returning from Iran. No clinical data is available, nor any additional lab-results. The following photos show structures that were found in the patient’s stools. The average size of these objects is 42 x 25 µm. Click on the images to enlarge.
What is the diagnosis (& possible clinical relevance)?
What is the diagnosis (& possible clinical relevance)?
Sunday, December 2, 2018
Answer to Case 521
Answer: Dicrocoelium dendricitum eggs
This is relatively rare find in human stool specimens. The eggs are small (35-45 µm long by 20-30 µm wide), thick-walled, and often brown due to bile staining. They are shed in a fully embryonated state, with the embryo often easily seen within the egg (these look like a skull to me!)
What this nice narrative does not mention are the following fun facts:
1. While in the ant intermediate host, the parasite takes control of the ant's nervous system and controls its actions. It directs the ant to climb to the top of a blade of grass every night and clamp down tightly to the blade with its mandibles. At dawn, the ant goes back to its normal activity in its colony. The recurs night after night, with the poor parasitized ant residing on the blade of grass until it is eaten by the grazing definitive host! What a clever way for a parasite to ensure the continuation of its own life cycle. This story reminds me of the effect that Toxoplasma gondii has on its rodent host (a story for another day).
2. As Old One reminded us, "D.d is beautiful critter. So flat you can clearly see all it's major organ systems without the benefit of stains. So thin it is commonly called the Lancet fluke. These dimensions allow D.d to dwell within the biliary ducts where they can allow normal bile flow in small numbers or block the flow with greater numbers." For this reason, the fluke is less likely to cause symptoms in its host.
3. When humans serve as the definitive host for D. dendricitum, eggs of the parasite may be seen in the stool. However, eggs may also be seen in the stool when humans inadvertently eat the infected liver of another definitive host such as a cow; in this scenario, the presence of eggs would be considered spurious passage or 'pseudo-parasitism'.
This brings us to the question that Idzi posed to us: What is the possible clinical relevance of finding these eggs in a human stool specimen? Luis and Nema both pointed out that it is necessary to rule out spurious egg passage due to ingestion of infected liver. To accomplish this Nema suggested collecting repeat stool specimens (instructing the patient not to eat any more liver!) If follow-up specimens are negative, then this would provide evidence that the patient was not actually parasitized. A related note is that liver ingestion is not common in many parts of the world, so getting a good dietary history (including possible ingestion of ants!) is very helpful.
I'll now leave you with a lovely story from Blaine:
So, there is this old story, or so I am told, about ants getting ready for the winter. They gathered up food stores all day, including slime balls produced by snails. Then along came a lazy grasshopper, which had spent the summer playing and not preparing for the winter. When the winter came, the grasshopper begged the ants for something to eat, even a slime ball!!! The ants refused. In the Spring, the ants had an insatiable urge to climb to the tops of blades of grass and ended up getting eaten by cattle and sheep! The grasshopper did not get eaten by the livestock. The moral of this story? Play all day and you are guaranteed not to become cow chow!
This is relatively rare find in human stool specimens. The eggs are small (35-45 µm long by 20-30 µm wide), thick-walled, and often brown due to bile staining. They are shed in a fully embryonated state, with the embryo often easily seen within the egg (these look like a skull to me!)
D. dendricitum is such a fascinating parasite. Its life cycle typically involves as ruminant, a snail and an ant. The whole life cycle is nicely illustrated by the CDC DPDx group HERE. Here is the accompanying text:
Ruminants are the usual definitive hosts for Dicrocoelium dendricitum, although other herbivorous animals, carnivores, and humans can serve as definitive hosts. Embryonated eggs are shed in feces. The eggs are ingested by a snail. Many species of snail may serve as the first intermediate host, including Zebrina spp. and Cionella spp. When the miracidia hatch, they migrate through the gut wall and settle into the adjacent vascular connective tissue, where they become mother sporocysts. The sporocysts migrate to the digestive gland where they give rise to several daughter sporocysts. Inside each daughter sporocyst, cercariae are produced. The cercariae migrate to the respiration chamber where they are shed in slime ball from the snail. After a slime ball is ingested by an ant, the cercariae become free in the intestine and migrate to the hemocoel where they become metacercariae. Many ants may serve as the second intermediate host, especially members of the genus, Formica. After an ant is eaten by the definitive host, the metacercariae excyst in the small intestine. The worms migrate to the bile duct where they mature into adults. Humans can serve as definitive hosts after accidentally ingesting infected ants.
What this nice narrative does not mention are the following fun facts:
1. While in the ant intermediate host, the parasite takes control of the ant's nervous system and controls its actions. It directs the ant to climb to the top of a blade of grass every night and clamp down tightly to the blade with its mandibles. At dawn, the ant goes back to its normal activity in its colony. The recurs night after night, with the poor parasitized ant residing on the blade of grass until it is eaten by the grazing definitive host! What a clever way for a parasite to ensure the continuation of its own life cycle. This story reminds me of the effect that Toxoplasma gondii has on its rodent host (a story for another day).
2. As Old One reminded us, "D.d is beautiful critter. So flat you can clearly see all it's major organ systems without the benefit of stains. So thin it is commonly called the Lancet fluke. These dimensions allow D.d to dwell within the biliary ducts where they can allow normal bile flow in small numbers or block the flow with greater numbers." For this reason, the fluke is less likely to cause symptoms in its host.
3. When humans serve as the definitive host for D. dendricitum, eggs of the parasite may be seen in the stool. However, eggs may also be seen in the stool when humans inadvertently eat the infected liver of another definitive host such as a cow; in this scenario, the presence of eggs would be considered spurious passage or 'pseudo-parasitism'.
This brings us to the question that Idzi posed to us: What is the possible clinical relevance of finding these eggs in a human stool specimen? Luis and Nema both pointed out that it is necessary to rule out spurious egg passage due to ingestion of infected liver. To accomplish this Nema suggested collecting repeat stool specimens (instructing the patient not to eat any more liver!) If follow-up specimens are negative, then this would provide evidence that the patient was not actually parasitized. A related note is that liver ingestion is not common in many parts of the world, so getting a good dietary history (including possible ingestion of ants!) is very helpful.
I'll now leave you with a lovely story from Blaine:
So, there is this old story, or so I am told, about ants getting ready for the winter. They gathered up food stores all day, including slime balls produced by snails. Then along came a lazy grasshopper, which had spent the summer playing and not preparing for the winter. When the winter came, the grasshopper begged the ants for something to eat, even a slime ball!!! The ants refused. In the Spring, the ants had an insatiable urge to climb to the tops of blades of grass and ended up getting eaten by cattle and sheep! The grasshopper did not get eaten by the livestock. The moral of this story? Play all day and you are guaranteed not to become cow chow!
Subscribe to:
Posts (Atom)